Systemic Joint Products: Straight to the Blood Supply
In the second article of this three-part series on joint supplements, Clinical Assistant Professor of Sports Medicine and Surgery at Virginia Tech's Marion duPont Scott Equine Medical Center (EMC) Dr. Maureen Kelleher examines the different systemic joint products horse owners have to choose from. Click here to read part one, which covers joint anatomy and disease.
It is easy to become overwhelmed by the choices when choosing among different joint products. There are FDA-approved injectable drugs, including those that are injected directly into the joint intra-articularly (IA), or as intravenous (IV) and intramuscular (IM) injections. There are also non-FDA-approved medical devices, as well as a myriad of different oral supplements to choose from – all of which have a different mechanism of action within the joint. With the whole wide world of joint products available to horse owners, how does one decide which is the right choice?
After detailing the anatomy of the joint, Dr. Maureen Kelleher turned to a discussion of the physiology of joint function, the changes that occur in degenerative joint disease, and the different options available to horse owners to treat degenerative joint disease, also called osteoarthritis. “Now that we know the anatomy of the joint and how it becomes diseased, we can talk about how we can potentially treat it.” Kelleher focused first on systemic (intravenous and intramuscular) products, followed by a non-FDA-approved medical device and oral supplements.
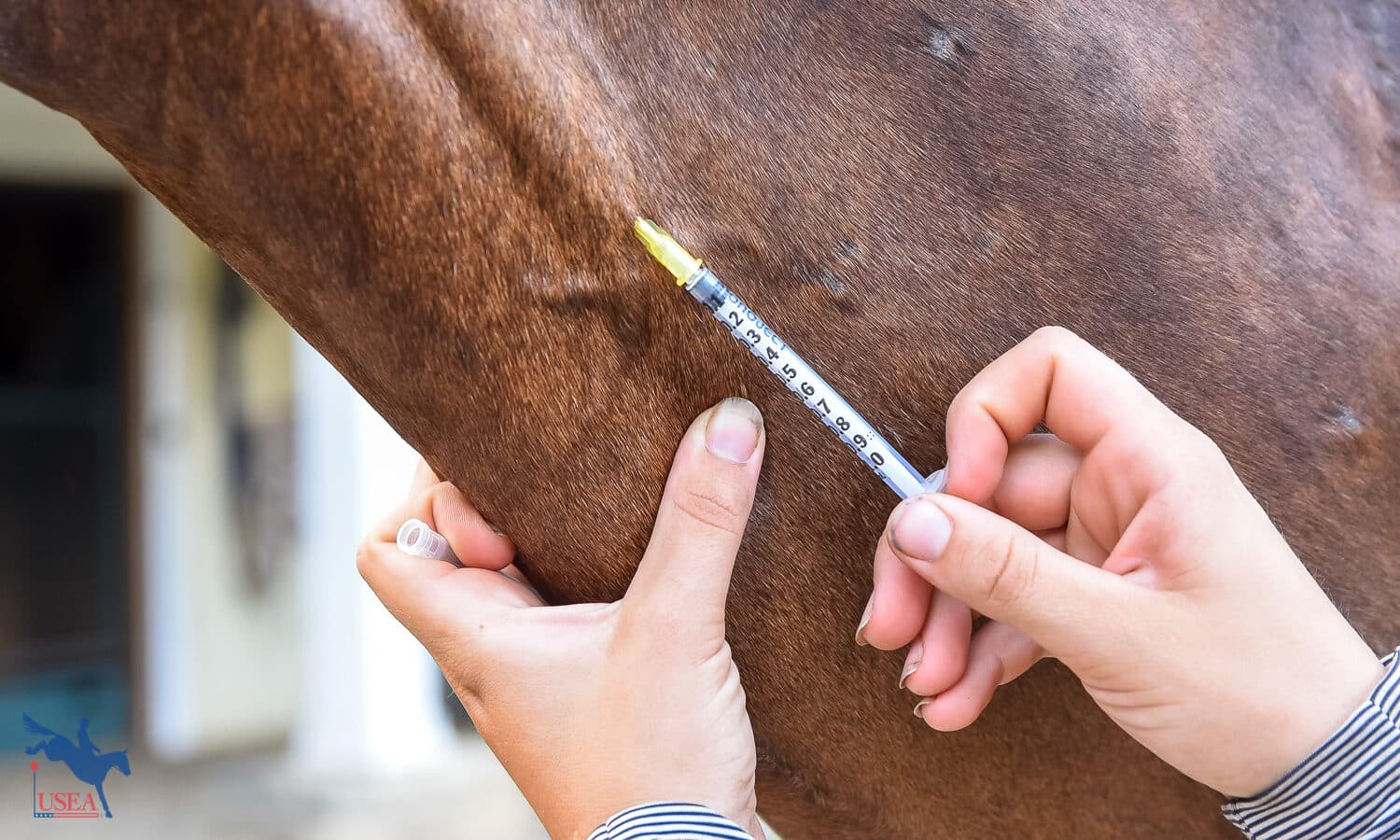
Intravenous and intramuscular injectable products are often called systemic treatments, meaning they are available to the whole body once administered. “When [injected into] the horse, it doesn’t just go to one joint; it goes to any of the joints it wants to,” Kelleher explained.
The single type of intravenous injection for treating joint disease is hyaluronic acid. “Hyaluronic acid is intended to trigger the synovial membrane to produce better-quality hyaluronan and improve the lubrication within the joint. There is only one FDA-approved intravenous hyaluronic acid product available for horses, and that is Legend.”
In comparison, polysulfated glycosaminoglycan (PSGAG) is administered via intramuscular injection. “Polysulfated glycosaminoglycan promotes the production of glycosaminoglycans (GAGs), hyaluronan (HA), and collagen within the joint and works to inhibit the breakdown of these products. By administering this, we’re trying to mimic the actual effect of GAGs in the joint.” Like hyaluronic acid, there is only one FDA-approved intramuscular polysulfated glycosaminoglycan product on the market: Adequan® i.m.

Kelleher took a moment to address Pentosan, a non-FDA-approved medical device which is frequently compared to Adequan. Pentosan polysulfate is a human medicine used to treat clot formation and relieve bladder inflammation. “It has a structure that is similar to polysulfated glycosaminoglycan and so the proposed mechanism is that it acts like PSGAG and encourages the production of GAGs, hyaluronan, and collagen and discourages their breakdown.”
However, Pentosan is not approved by the FDA for use in horses. Use of Pentosan as a drug for osteoarthritis is considered non-approved use of a medical device. There are several similar products on the market, none of which are FDA-approved for intravenous or intramuscular use in horses, and Kelleher cautioned against their use. “Can you use them when they are not FDA-approved? Absolutely. However, there is no support for safety or efficacy when using medical devices as if they are FDA-approved drugs.”
“Yes, Adequan and Legend are more expensive,” she acknowledged, “and they’re more expensive because they went through the effort of obtaining FDA approval to show they work and are safe in horses for their intended use [either intramuscularly or intravenously].”
Outside of the FDA-approval studies, Kelleher explained that there is not a large body of independent controlled scientific research on Legend or Adequan and the expected response to treatment may vary by horse. “Whether you will see a significant difference in your horse is hard to say,” she stated. “There are a high percentage of equine veterinarians that use and recommend Adequan and Legend in horses that have osteoarthritis to help mitigate the effects of continued degeneration.”
What about the wide variety of oral equine joint supplements available to horse owners? Stay tuned for the third and final installment in which Dr. Kelleher reviews oral supplements.
About Dr. Maureen Kelleher
Dr. Maureen Kelleher earned her Doctor of Veterinary Medicine at the University of California, Davis, in 2006. She then completed an internship at Pioneer Equine Hospital in Oakdale, California, and a residency in equine surgery at University of California, Davis. Before joining EMC, Dr. Kelleher gained years of experience in equine private practice in California with a focus on equine sports medicine and lameness, advanced diagnostic imaging, and acupuncture. She became a certified veterinary acupuncturist in 2010, and earned Diplomate status with the American College of Veterinary Surgeons in 2013. Dr. Kelleher focuses on the assessment and non-surgical treatment of performance-limiting problems in sport horses. She works closely with EMC’s therapeutic farrier team and its medicine and surgery teams, utilizing advanced diagnostic imaging capabilities to provide equine patients with superior care.















